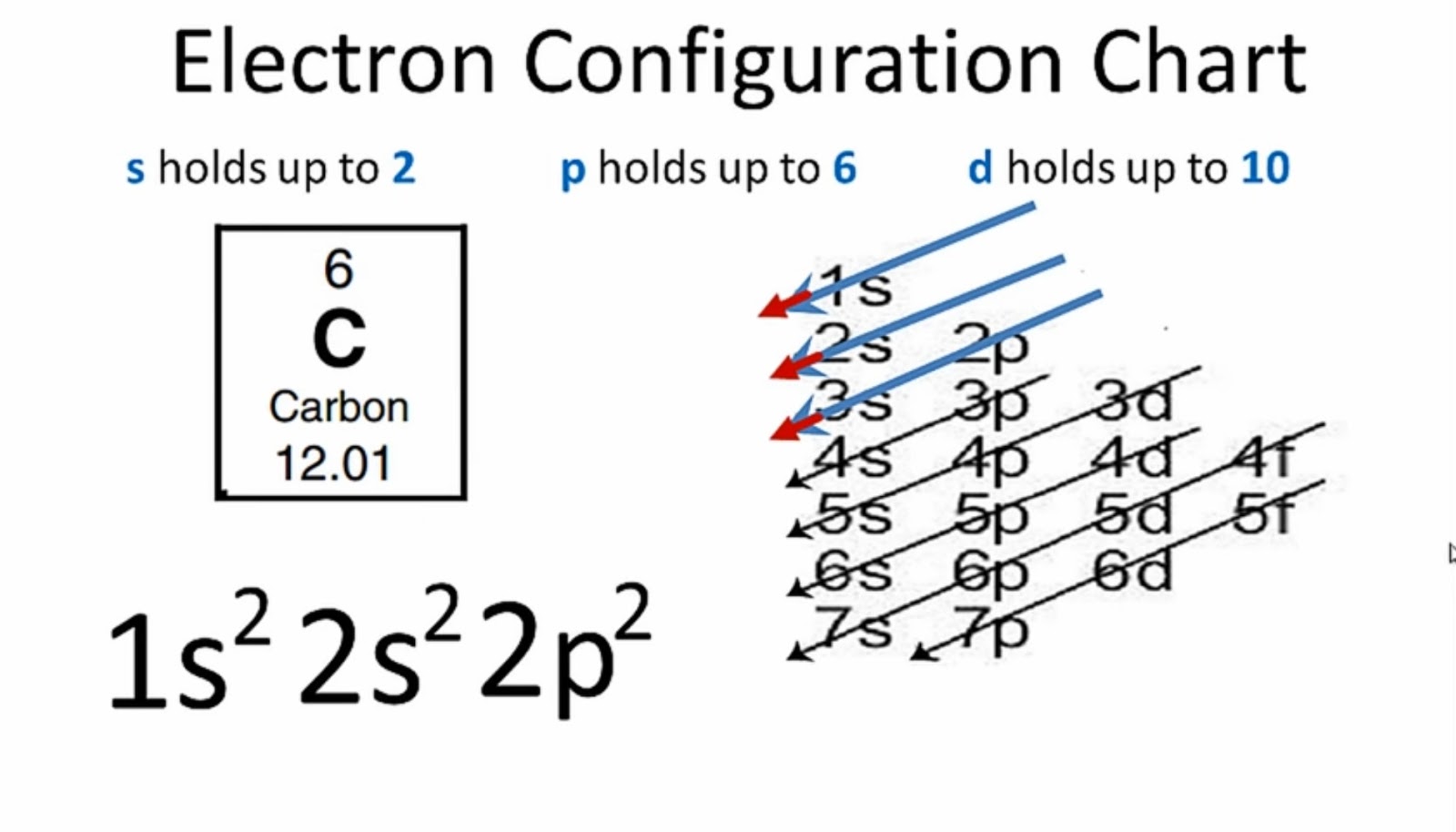
The arrangement of electrons within a carbon atom is fundamental to its chemical behavior. This distribution dictates how carbon interacts with other elements, forming the vast array of organic molecules essential to life. Carbon's atomic number is 6, meaning it possesses 6 electrons. The electronic configuration, often written as 1s22s22p2, specifically details the arrangement of these electrons in different energy levels and sublevels within the atom. This configuration, with two electrons in the 1s orbital, two in the 2s orbital, and two in the 2p orbital, underlies the exceptional bonding properties of carbon.
Carbon's unique electronic configuration allows it to form four strong covalent bonds with other atoms. This tetravalency is a key characteristic enabling carbon to bond with a wide variety of elements, including hydrogen, oxygen, nitrogen, and many others. This ability to form diverse structures, from simple chains to complex ring systems, is crucial to the existence of organic compounds, which are the basis of all known life forms. The resulting molecules demonstrate a remarkable diversity of functions, ranging from the formation of structural components to the storage of genetic information.
Understanding this electronic structure is crucial to comprehending the principles behind organic chemistry, material science, and biological processes. The subsequent discussion will delve into the specifics of carbon-based bonding, highlighting the different types of carbon-carbon interactions and their implications.
Carbon Electronic Configuration
The arrangement of electrons within a carbon atom profoundly influences its chemical behavior and forms the basis of its remarkable versatility in bonding and molecular diversity.
- Atomic Number (6)
- Electron Arrangement (1s22s22p2)
- Four Valence Electrons
- Tetravalency
- Covalent Bonding
- Hybridization
- Organic Molecules
- Life's Building Block
The core aspects of carbon's electronic configuration, such as its atomic number of six, define its electron arrangement (1s22s22p2). This arrangement leads to four valence electrons, enabling carbon to form four covalent bonds. Hybridization of these orbitals allows the creation of diverse structures, crucial for organic molecules and ultimately, biological systems. The tetravalency of carbon is essential for building complex molecules like proteins, carbohydrates, and DNA. Understanding these fundamental principles is key to comprehending the vast array of carbon-based molecules that constitute life as we know it.
1. Atomic Number (6)
The atomic number of carbon, six, dictates the fundamental structure of the atom and, consequently, its electronic configuration. This number signifies the presence of six protons in the carbon nucleus, directly influencing the number of electrons orbiting the nucleus. The balance between protons and electrons in a neutral atom is crucial for understanding its bonding potential and, in the case of carbon, its remarkable capacity to form diverse molecules.
- Electron Count and Arrangement
The atomic number directly determines the number of electrons present in a neutral carbon atom. With six electrons, the electronic configuration, 1s22s22p2, arises from the specific filling of atomic orbitals based on energy levels and Hund's rule. This fundamental arrangement is the basis for carbon's chemical reactivity.
- Foundation for Bonding Capacity
The configuration, driven by the atomic number's influence on electron distribution, determines carbon's unique valence electron count (four). This tetravalency enables carbon's ability to form four covalent bonds with other atoms, a critical feature enabling the creation of diverse organic molecules. This four-bond capacity distinguishes carbon from most other elements.
- Determinant of Molecular Shapes
Carbon's atomic number (six) has a bearing on the types of molecular structures that can be formed. Different arrangements of carbon atoms, due to the spatial orientation of bonds, lead to different shapes and properties in resulting molecules. This structural diversity is essential in organic chemistry and material science.
- Implications in Biological Systems
The influence of the atomic number on electron configuration is fundamental to biological processes. The extensive diversity of organic moleculesproteins, carbohydrates, and nucleic acidsare built upon carbon atoms' capacity for forming stable and varied bonds based on the principles arising from the atomic number of six.
In conclusion, the atomic number of carbon (six) establishes the basis for its electron configuration, ultimately determining its exceptional bonding capacity. This, in turn, dictates the vast array of organic molecules, making it essential to life itself. The specifics of carbon's bonding behaviors stem directly from this fundamental characteristic.
2. Electron Arrangement (1s22s22p2)
The notation 1s22s22p2 meticulously describes the arrangement of electrons within a carbon atom. This arrangement, a cornerstone of carbon's electronic configuration, dictates the atom's behavior in chemical reactions. The superscripts represent the number of electrons occupying each atomic orbital. The 1s orbital, closest to the nucleus, holds two electrons. Next, the 2s orbital also accommodates two electrons. Finally, the 2p orbital, higher in energy and with three sub-orbitals, has two electrons distributed according to Hund's rule, maximizing the number of unpaired electrons.
This specific arrangement directly influences carbon's ability to form chemical bonds. The four valence electronstwo from the 2s orbital and two from the 2p orbitalsare crucial in this process. These four valence electrons allow carbon to readily share electrons with other atoms, resulting in the formation of strong covalent bonds. This feature underpins the vast diversity of organic molecules. For instance, the single covalent bonds in methane (CH4) arise from the sharing of electrons between carbon's valence electrons and the valence electrons of four hydrogen atoms. The ability to form multiple bonds, like double bonds in ethene (C2H4), further highlights the significance of this electronic structure.
Understanding the electron arrangement (1s22s22p2) is fundamental to comprehending the chemistry of carbon. It explains carbon's remarkable capacity to form diverse structures and its crucial role in organic chemistry. This knowledge is critical in various applications, from designing new materials with specific properties to understanding biological processes. The versatility of carbon-based molecules, enabling the creation of countless organic compounds and their unique structures, stems directly from this particular electronic arrangement within the carbon atom.
3. Four Valence Electrons
The presence of four valence electrons is a direct consequence of carbon's electronic configuration. The configuration 1s22s22p2 dictates that four electrons occupy the outermost energy level (n=2). These four electrons, termed valence electrons, are the most loosely held and thus are readily involved in chemical bonding. This characteristic is paramount to carbon's versatility.
The availability of four valence electrons allows carbon to form four covalent bonds with other atoms. This tetravalency is central to the formation of vast arrays of organic molecules. For instance, in methane (CH4), carbon forms four single bonds with four hydrogen atoms. In ethane (C2H6), each carbon atom forms four bonds, with two of those bonds connecting the carbon atoms themselves, showcasing the ability to create chains and complex structures. Similarly, the carbon atoms in benzene (C6H6) form a stable ring structure through alternating single and double bonds, a consequence of the characteristic ability to form multiple bonds, again facilitated by these four valence electrons.
Understanding the relationship between four valence electrons and carbon's electronic configuration is crucial in various fields. In organic chemistry, it explains the vast diversity of carbon-based molecules. In materials science, it informs the design of new carbon-based materials with tailored properties. The ability of carbon to form these diverse structures enables the creation of polymers, pharmaceuticals, and countless materials essential for modern technology. This understanding provides a framework for comprehending the intricate chemistry underpinning biological processes and numerous technological applications.
4. Tetravalency
Carbon's tetravalency, the capacity to form four covalent bonds, is inextricably linked to its electronic configuration. This characteristic, crucial to the vast diversity of organic molecules, stems directly from the atom's electron arrangement. Understanding this relationship reveals the fundamental principles governing carbon's bonding behavior.
- Orbital Hybridization
Carbon's electronic configuration, specifically 1s22s22p2, provides the foundation for tetravalency. In many cases, the 2s and 2p orbitals hybridize, creating four equivalent sp3 hybrid orbitals. This hybridization is critical; it facilitates the formation of four identical bonds, a direct consequence of the electron arrangement in the valence shell.
- Covalent Bonding Mechanism
These hybridized orbitals permit carbon to share electrons with other atoms, forming strong covalent bonds. This sharing of electrons satisfies the octet rule for carbon, achieving a stable electron configuration. The strength and directionality of these bonds are determined by the arrangement of these hybridized orbitals, enabling a wide array of molecular geometries and, thus, diverse organic compounds.
- Diverse Molecular Structures
Tetravalency allows carbon to form a vast array of molecular structures. Chains, branched structures, rings, and complex three-dimensional arrangements are possible. The ability to form single, double, and triple bonds with other carbon atoms, as well as with various other elements, amplifies the possibilities, from simple hydrocarbons to intricate biological molecules.
- Impact on Functional Groups
Tetravalency is pivotal in defining functional groups, which are specific arrangements of atoms that impart characteristic chemical behaviors to molecules. Functional groups like alcohols, carboxylic acids, and amines, all featuring carbon atoms exhibiting tetravalency, dictate how molecules interact and react. This diversity in functional groups is essential for the complexity and functionality of organic molecules.
In summary, carbon's tetravalency arises directly from its electronic configuration, specifically the availability of four valence electrons and the subsequent hybridization of atomic orbitals. This feature underpins the remarkable versatility of carbon in forming diverse molecular structures and functional groups, a cornerstone of organic chemistry and the foundation of life's complexity.
5. Covalent Bonding
Covalent bonding is a fundamental interaction crucial for the behavior of carbon. The nature of carbon's electronic configuration directly influences its propensity for covalent bonding. Carbon, possessing four valence electrons, readily shares these electrons with other atoms to achieve a stable electron configuration, mirroring the octet rule. This sharing creates a covalent bond, where atoms collectively hold shared electrons. This shared electron cloud creates a strong attractive force between the nuclei, forming molecules. The ability to form multiple covalent bonds, including single, double, and triple bonds, further highlights this characteristic. Carbons ability to participate in covalent bonds is a direct consequence of its electronic configuration.
The implications of covalent bonding in carbon are profound. Consider methane (CH4): Carbons four valence electrons form single covalent bonds with four hydrogen atoms. This arrangement satisfies the octet rule for both carbon and hydrogen. Similarly, in ethane (C2H6), carbon atoms form single covalent bonds with each other and with hydrogen atoms. These examples demonstrate how the electronic configuration, enabling covalent bonding, allows the creation of various stable organic molecules. The flexibility and strength of covalent bonding in carbon-based systems are essential for the formation of complex molecules, including polymers, proteins, and DNA, crucial components of living organisms. This understanding is critical in the design and synthesis of new carbon-based materials with tailored properties.
In summary, carbon's electronic configuration, specifically its four valence electrons, dictates its propensity for covalent bonding. This type of bonding allows the formation of a vast array of organic compounds with diverse structures and properties. The stability and strength of these covalent bonds are essential to the formation of complex molecules, including those critical for life itself. Understanding the interplay between covalent bonding and carbon's electronic configuration is pivotal in various fields, including organic chemistry, material science, and biotechnology.
6. Hybridization
Hybridization, a crucial concept in chemistry, significantly impacts the properties and behavior of carbon, stemming directly from its electronic configuration. Specifically, the electronic configuration of carbon, with its four valence electrons, necessitates a mechanism for accommodating the formation of multiple, stable bonds. Hybridization addresses this need by redistributing electron density in atomic orbitals, resulting in new hybrid orbitals. This process fundamentally alters the bonding characteristics of carbon.
The electronic configuration of carbon, 1s22s22p2, initially suggests that carbon can form only four bonds. However, carbon's ability to form diverse structures, from simple alkanes to complex molecules like proteins, necessitates more than four simple bonds. Hybridization provides the solution. Through the mixing of atomic orbitals (one 2s orbital and three 2p orbitals), carbon can generate four new, equivalent sp3 hybrid orbitals. These hybrid orbitals have different shapes and energies than the original atomic orbitals, allowing carbon to form four identical sigma () bonds with other atoms. This explains why methane (CH4) has a tetrahedral shape; the four sp3 hybrid orbitals arrange themselves tetrahedrally around the carbon atom. Similarly, the double bond in ethene (C2H4) involves the formation of sp2 hybrid orbitals by carbon, with the remaining p orbital forming a pi () bond. The sp hybridization, seen in acetylene (C2H2), allows for the formation of triple bonds.
Understanding hybridization is essential in various scientific fields. In organic chemistry, it explains the diverse structures and properties of organic molecules. In materials science, understanding hybrid orbitals helps in the design of new materials with tailored properties. In biochemistry, it's crucial to comprehend the intricate three-dimensional structures of proteins and other biological molecules, which rely heavily on carbon's ability to form various hybrid orbitals. The ability to predict and understand the spatial arrangements of atoms within molecules is fundamental to explaining their reactivity and ultimately their function. The connection between carbon's electronic configuration and hybridization is a powerful example of how fundamental principles dictate the complexity of molecular structures and interactions.
7. Organic Molecules
Organic molecules, the cornerstone of life, exhibit a remarkable diversity of structures and functionalities. This diversity is inextricably linked to the electronic configuration of carbon. Carbon's unique ability to form four covalent bonds, a direct consequence of its four valence electrons, allows for the creation of a vast array of carbon-carbon and carbon-hydrogen chains, branched structures, and cyclic arrangements. This versatility permits the construction of complex molecular scaffolds capable of hosting a multitude of functional groups. The electronic configuration of carbon dictates the bonding patterns and ultimately the structural integrity of organic molecules.
The tetravalency of carbon, a direct consequence of its electronic configuration (1s22s22p2), is fundamental to the formation of diverse organic molecules. Consider hydrocarbons, a group of organic compounds composed solely of carbon and hydrogen. The myriad forms of hydrocarbons, from simple alkanes to complex aromatic systems, arise directly from variations in carbon-carbon bonding and chain structures. Similarly, biological macromolecules like proteins, carbohydrates, and nucleic acids, the essential components of life, rely on carbon's capacity for bonding. Proteins, for example, are intricate chains of amino acids, wherein carbon plays a crucial role in forming the backbone of the molecule and attaching various functional groups, thereby dictating the protein's specific structure and function. The unique electronic configuration of carbon enables this precise arrangement, establishing the diversity of organic molecules essential for life's processes.
Understanding the connection between organic molecules and carbon's electronic configuration has profound practical significance. The synthesis of new pharmaceuticals and materials relies on this knowledge. The design of new drugs, for instance, often involves modifying existing organic molecules to enhance their therapeutic efficacy, and knowledge of carbon's electronic structure is key to predicting the behavior and properties of these modified molecules. In the realm of materials science, understanding carbon's unique ability to form complex structures is crucial for creating novel materials with specialized properties. The design of advanced polymers, carbon nanotubes, and other carbon-based materials heavily relies on the insights derived from carbon's electronic configuration. In essence, mastery of this fundamental link unlocks the potential to design, synthesize, and manipulate a wide range of organic molecules, leading to advancements across various scientific and technological disciplines.
8. Life's Building Block
The fundamental building block of life is intricately connected to carbon's electronic configuration. Carbon's unique ability to form diverse, stable molecules arises directly from its electronic structure. This structure enables carbon to form four strong covalent bonds with a wide array of other elements, including hydrogen, oxygen, nitrogen, and phosphorus. This tetravalency is crucial because it allows carbon to create a vast array of molecular structures, from simple chains to complex three-dimensional networks. These structures are the basis of all known organic molecules, including the essential components of living organisms. The specific arrangement of electrons in carbon's outermost shell, the valence shell, dictates how it bonds with other atoms. The resultant molecular shapes and interactions dictate the functionality of these molecules.
The significance of this relationship is profound. The ability to form complex, stable molecules is essential for life's processes. Proteins, crucial for structure and function in living organisms, are composed of amino acids linked by peptide bonds, formed by carbon. Carbohydrates, providing energy and structural support, are constructed using carbon backbones. Deoxyribonucleic acid (DNA) and ribonucleic acid (RNA), carrying genetic information, utilize carbon's bonding versatility to form the essential sugar-phosphate backbones and nitrogenous bases. Without carbon's ability to form these diverse molecules, life as we know it would not be possible. The precise nature of these molecules depends entirely on the ability of carbon to form varied bonds due to its electronic configuration.
Understanding this connection between life's building blocks and carbon's electronic configuration has significant practical implications. It provides a framework for understanding the mechanisms of life processes. Further, it drives research in areas like drug development, where the ability to modify organic molecules to target specific biological pathways hinges on knowledge of carbon's bonding behaviors, dictated by its electron configuration. The design of new materials and technologies, often incorporating carbon-based structures, is also deeply rooted in this fundamental relationship. A deep understanding of carbon's electron arrangement provides valuable tools for predicting molecular behavior and creating new molecules with specific characteristics.
Frequently Asked Questions about Carbon Electronic Configuration
This section addresses common inquiries surrounding carbon's electronic configuration, aiming to clarify key aspects and dispel potential misconceptions. A comprehensive understanding of this topic is fundamental to grasping the principles of organic chemistry, material science, and biological systems.
Question 1: What is the electronic configuration of carbon, and why is it important?
Carbon's electronic configuration is 1s22s22p2. This arrangement signifies the distribution of electrons within the atom. Its importance stems from the four valence electrons (two in the 2s orbital, and two in the 2p orbitals). These valence electrons are crucial for carbon's ability to form four covalent bonds, a defining characteristic underpinning the immense diversity of organic molecules.
Question 2: How does carbon's electronic configuration relate to its tetravalency?
Carbon's tetravalency, its ability to form four bonds, is a direct consequence of its electronic configuration. The four valence electrons allow carbon to readily share electrons with other atoms, satisfying the octet rule and forming strong covalent bonds. Orbital hybridization, specifically sp3 hybridization, explains this capacity to form four equivalent bonds.
Question 3: What role does hybridization play in carbon's bonding?
Hybridization, specifically sp3, sp2, and sp hybridization, redistributes electron density in carbon's atomic orbitals. This process results in hybrid orbitals that facilitate the formation of strong, directional covalent bonds. Different hybridization types lead to different molecular geometries and, consequently, variations in the structures and properties of carbon-based molecules.
Question 4: Why is carbon so versatile in forming different types of bonds?
Carbon's ability to form single, double, and triple bonds with other atoms, including itself, is directly related to its electronic configuration and subsequent hybridization. The presence of four valence electrons and the capacity for different hybridization states allow for the creation of an extensive variety of molecular structures, contributing to the remarkable diversity of organic molecules.
Question 5: How does carbon's electronic configuration contribute to the formation of organic molecules?
Carbon's ability to form four bonds allows for the creation of extensive carbon-carbon chains, branches, and rings. These diverse structures provide the framework for the formation of a vast array of organic molecules. The specific arrangement of these carbon atoms, dictated by the electronic configuration, determines the overall structure and properties of the resulting molecules.
Question 6: What are some practical applications of understanding carbon's electronic configuration?
A deep understanding of carbon's electronic configuration has wide-ranging implications. It is crucial in organic chemistry for predicting and synthesizing new molecules. In materials science, it guides the development of new carbon-based materials with specific properties. Furthermore, in fields like biochemistry, it provides a foundation for comprehending the structural features and functions of complex biological molecules.
In conclusion, carbon's electronic configuration is fundamental to its versatility and the diversity of organic compounds. This understanding is essential for a wide range of scientific disciplines, including organic chemistry, material science, and biology.
The subsequent section will delve into the applications of carbon-based materials in diverse fields.
Tips for Understanding Carbon Electronic Configuration
This section provides practical guidance for comprehending carbon's electronic configuration, a cornerstone of organic chemistry and essential for understanding biological systems and materials science. These tips facilitate a deeper understanding of the fundamental principles governing carbon's behavior and its role in forming a vast array of molecules.
Tip 1: Master the Notation System. Understanding the notation, such as 1s22s22p2, is paramount. This notation signifies the electron distribution across different energy levels (shells) and sublevels (orbitals) within the atom. Grasping the principles behind orbital filling (e.g., Hund's rule and the Aufbau principle) is critical for accurate representation of the electronic configuration and its relationship to chemical behavior.
Tip 2: Recognize the Significance of Valence Electrons. Carbon's valence electrons are the electrons in the outermost shell (n=2 in the case of carbon). These electrons are directly involved in chemical bonding. Focusing on these electrons facilitates understanding of bonding patterns, molecular geometry, and overall reactivity.
Tip 3: Appreciate the Role of Hybridization. Carbon's ability to form multiple bonds (single, double, triple) is explained by orbital hybridization. Hybridization involves mixing atomic orbitals to form new hybrid orbitals, thereby enabling the formation of various bond types and molecular geometries. Understanding sp3, sp2, and sp hybridization is crucial for predicting and interpreting molecular structures.
Tip 4: Utilize Models to Visualize Electron Arrangement. Employing molecular models can be beneficial for visualizing the spatial arrangement of atoms in molecules. Models aid in understanding the relationship between electronic configuration, molecular geometry, and ultimately, the chemical properties of the compound.
Tip 5: Explore Common Organic Compounds. Studying the structures of common organic compoundsmethane (CH4), ethene (C2H4), and othersprovides concrete examples of how different hybridization states lead to distinct molecular shapes and properties. Comparative analyses of these compounds help to solidify the conceptual understanding of the relationship between electronic configuration and molecular behavior.
Tip 6: Consult Relevant Literature. Referencing reputable scientific literature, textbooks, and online resources provides a comprehensive overview of carbon's electronic configuration and its implications in various contexts. These external resources offer detailed explanations and examples not available in a single tip.
By applying these tips, a solid foundation for comprehending the nuances of carbon's electronic configuration can be established, leading to a clearer picture of carbon's crucial role in numerous chemical and biological processes. This understanding is vital for advancing research and innovation in diverse fields.
Subsequent sections will explore specific applications and implications of carbon's unique electronic configuration in greater detail.
Conclusion
Carbon's electronic configuration, specifically its 1s22s22p2 arrangement, underpins its exceptional chemical versatility. The four valence electrons dictate carbon's tetravalency, enabling the formation of an extraordinary diversity of covalent bonds. This characteristic allows for the creation of complex molecular structures, from simple hydrocarbons to intricate biological molecules. Hybridization plays a critical role in shaping the geometries and properties of these molecules. Understanding the interplay between electronic configuration, hybridization, and bonding patterns is fundamental to comprehending the underlying principles of organic chemistry, materials science, and biochemistry. The prevalence of carbon-based compounds in biological systems highlights the profound significance of this electronic configuration in supporting life's processes.
The exploration of carbon electronic configuration reveals a fundamental principle governing molecular diversity and the creation of complex systems. Further investigation into the specific interactions of carbon with other elements and the implications of this interaction within diverse contexts will continue to yield valuable insights. This knowledge is crucial for advancements in fields ranging from drug development and materials science to understanding the origin and evolution of life itself.